Close
Shop All:Lab-Aids
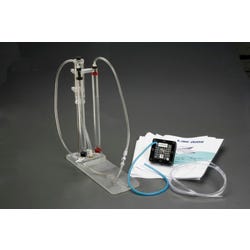
LAB-AIDS Hydrogen and Fuel Cells Investigating Alternative Energy
Item #:
1500961
$533.59
$266.80
In Stock - Typically Ships Within 2-3 Days
Free Shipping Eligible: Details
School Specialty Shipping Policy
Items Shipped Within the Contiguous 48 United States
Free Shipping Eligible Items
- Free shipping and handling on eligible supply orders of $69 or more. Free shipping calculation is based on the subtotals of eligible items, after any additional discounts are applied.
- For subtotals less than $69, the shipping and handling charge is $11.95.
Free Shipping Ineligible Items
- Shipping and handling charges are 20% of the subtotal of the items, after any discounts are applied, with a $11.95 minimum charge.
Free Shipping Ineligible Items
- Shipping and handling charges are 20% of the subtotal of the items, after any discounts are applied, with a $99 minimum charge. If the subtotal is greater than $1,000, please e-mail freight_quote@schoolspecialty.com for a freight quote.
*Note: Individually negotiated shipping policies will override the standard shipping policy. See Full Details
Items Shipped to Hawaii, Alaska and International
See our Hawaii & Alaska Shipping Policy and International Shipping Policy for details.
About This Item
Description
LAB-AIDS Investigating Alternative Energy: Hydrogen and Fuel Cells Kit measures the voltage/current produced by the fuel cell and are introduced to oxidation-reduction reactions and half-reactions as a means of creating electric current. It includes six activity modules that focus on hydrogen fuel cell technology as one potential solution to energy concerns. Students first consider some trade-offs of using various fuels, including hydrogen, for powering vehicles then use an electrolyser to produce hydrogen and oxygen gas from water. Students then use these gases to generate electricity with a proton exchange membrane fuel cell and observe the consumption of gases by the fuel cell and the motion of an electric propeller attached to it. To further their understanding of the chemistry involved, they use both a computer simulation and a manipulative model of a PEM fuel cell. To end the unit, students use the G of the reaction to compute the efficiency of the fuel cell in converting the energy in H2 to electricity then identify the advantages of and the challenges facing fuel cell powered vehicles.Features
Shipping Type:
parcel
Free Shipping:
true
Specifications
Certifications:
Not Applicable
Maximum Age:
12 years
Allergens:
Contains No Allergens